특징
- ioSensory Neuron은 opti-ox™ 기술을 사용하여 정밀하게 재프로그래밍된 감각세포입니다.
- revival 후 7일 이내에 pan-sensory neuron marker인 PRPH, BRN3A, ISL1 및 TUBB3 및 주요 마커의 발현을 특징으로 하는 통각수용체 정체성을 갖춘 매우 순수한(>99%) 감각 뉴런 집단을 형성합니다.
- TRPV1, TRPM3 및 TRPM8에 대한 선택적 작용제에 대한 반응성을 보이며, 기능성 통각수용체 표현형 (nociceptor phenotype)을 보여줍니다.
- 말초 신경병증에 대한 신뢰할 수 있는 만성 침해수용성 통증(chronic nociceptive pain) 연구 및 치료법 개발에 이용됩니다.
사양
| Starting
material |
Human iPSC line |
| Donor |
Caucasian adult male (skin
fibroblast) |
| Format |
Cryopreserved cells |
| Differentiation
method |
opti-ox™ cellular reprogramming |
| Karyotype |
Normal (46, XY) |
| Vial size |
Small: 2 x 10^6 viable cells |
| Recommended
seeding density |
60,000 cells/cm2 |
| Seeding
compatibility |
6, 12, 24, 96 and 384 well plates |
| Quality
control |
Sterility, protein expression (ICC),
gene expression (RT-qPCR) |
프로토콜
ioSensory Neuron은 냉동 보존 형식으로 제공되며 권장 배지에서 재생 시 빠르게 성숙되도록 프로그래밍되어 있습니다.
Phase 0 단계의 세포를 수령 후, Phase 1, 2 과정을 거쳐 실험에 바로 이용합니다.
1) Phase 0 : Induction (bit.bio에서 Induction 후 제공)
2) Phase 1 : 고객이 세포를 수령 후 plating 하여 안정화시키는 기간 (7일)
3) Phase 2 : 세포가 성숙하는 기간
참고 데이터
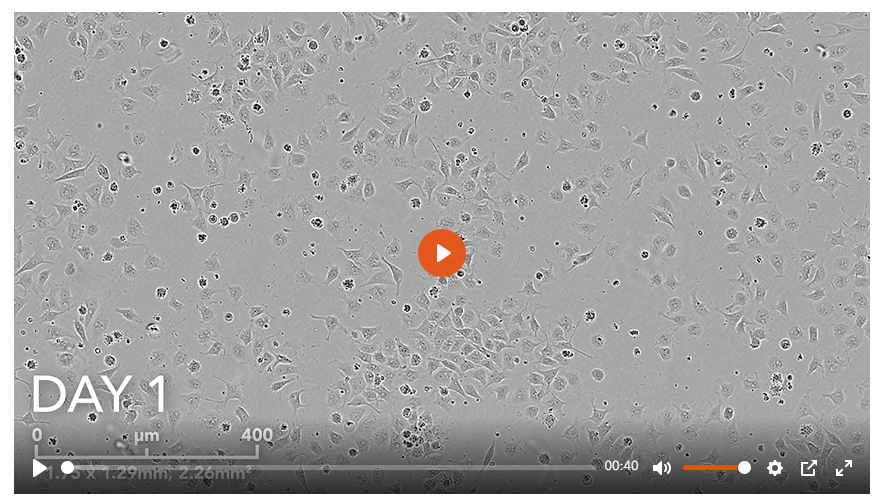
1. opti-ox precision reprogrammed ioSensory Neurons rapidly form a homogenous neuronal population
냉동보존된 ioSensory Neuron의 해동 후, 신속하고 균질하게 뉴런세포로 성장하는 과정을 저속 촬영한 14일간의 타임랩스 비디오입니다.
2. ioSensory Neurons express pan-sensory neuron-specific markers
revival 후 14일째에 ioSensory 뉴런의 면역형광 염색 이미지 입니다. 상단 패널은 ioSensory 뉴런이 BRN3A(빨간색), 범뉴런 마커 MAP2(녹색) 및 DAPI 대조염색(파란색)에 대해 양성임을 보여주며, 모든 MAP2 양성 뉴런이 감각 뉴런 정체성을 가지고 있음을 보여줍니다. 하단 패널은 ioSensory 뉴런이 ISL1(자홍색), PRPH(빨간색), 범뉴런 마커 TUBB3(녹색) 및 DAPI 대조염색(파란색)에 대해 양성임을 보여줍니다.

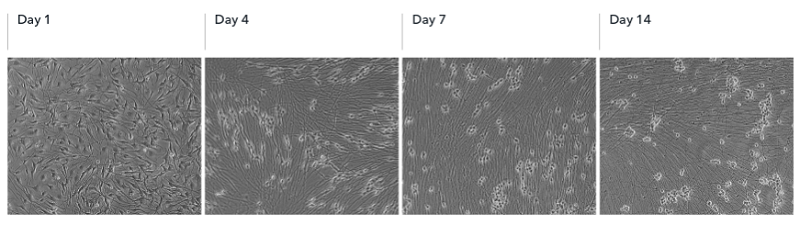
3. ioSensory Neurons show visible neuronal networks by day 7
리프로그래밍 시 세포에서 급격한 형태학적 변화가 관찰되며, 뉴런은 revival 후 4일째에 확인됩니다. 눈에 보이는 신경망은 해동 후 7일째에 관찰됩니다.
4. Single cell RNA-sequencing shows ioSensory Neurons form a pure population (>99%) of sensory neurons
단일 세포 RNA 서열 분석은 ioSensory Neuron을 사용하여 4가지 특정 시점(7일, 10일, 14일 및 17일)에 수행되었습니다. 7일째까지, 집단은 유사분열 후 감각 뉴런의 순수 집단(>99%)을 나타내는 뚜렷한 발현 프로파일을 갖습니다. 유전자 발현은 10x Genomics scRNA 시퀀싱으로 평가되었습니다.

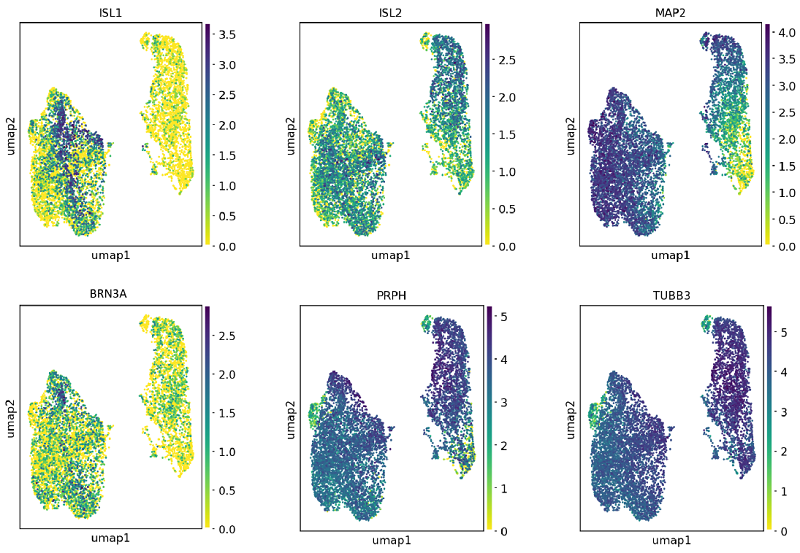
5. Single cell RNA-sequencing shows ioSensory Neurons express key pan-sensory neuronal markers
단일 세포 RNA 서열 분석은 ioSensory Neuron을 사용하여 4가지 특정 시점(7일, 10일, 14일 및 17일)에 수행되었습니다. 7일째에는 범감각 뉴런 마커 유전자인 ISL1, ISL2, PRPH, BRN3A와 범뉴런 마커인 TUBB3 및 MAP2의 발현이 유사분열 후 감각 뉴런에서 검출될 수 있었습니다. 유전자 발현은 10x Genomics scRNA 시퀀싱으로 평가되었습니다.
6. Single cell RNA-sequencing shows ioSensory Neurons express key nociceptor markers
단일 세포 RNA 서열 분석은 ioSensory Neuron을 사용하여 4가지 특정 시점(7일, 10일, 14일 및 17일)에 수행되었습니다. 7일째에는 유사분열 후 감각 뉴런에서 주요 통각수용기 표지 유전자(NTRK1 및 TRPV1)의 발현이 검출될 수 있습니다. 유전자 발현은 10x Genomics scRNA 시퀀싱으로 평가되었습니다.

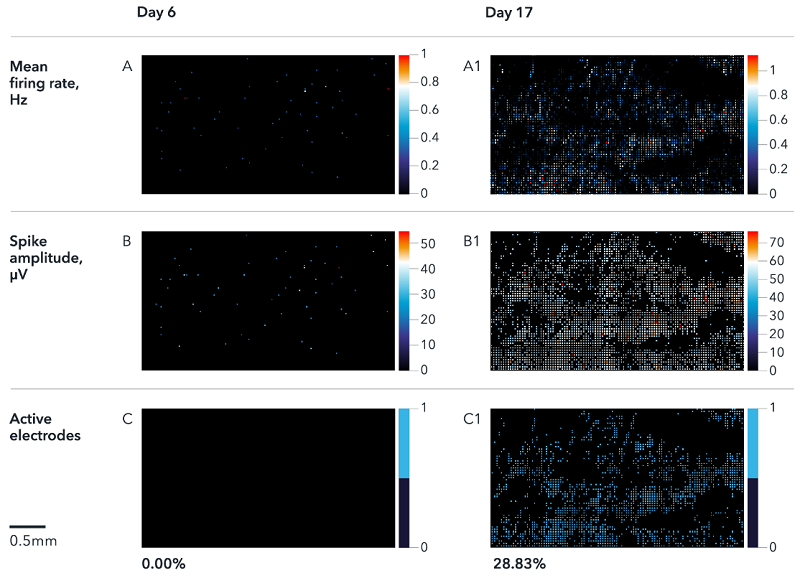
7. ioSensory Neurons display spontaneous activity that matures over time
6일과 17일에 ioSensory Neuron의 다중 전극 배열(MEA) 기록을 보여줍니다. Activity map에는 firing rate(A), spike 진폭(B) 및 활성 전극의 %(C)가 표시됩니다. 결과는 6일부터 17일까지 신경 성숙 동안 시간에 따른 자발적인 활동 증가를 보여줍니다. 분석은 Maxwell Biosystem의 MaxTwo 다중 웰 시스템에서 수행되었습니다.
8. ioSensory Neurons display a functional nociceptor phenotype
TRPV1(캡사이신), TRPM3(CIM0216) 및 TRPM8(WS-12)과 같은 온도 민감성 TRP 채널을 표적으로 하는 약리학적 작용제로 ioSensory Neuron을 자극한 Calcium mobilisation imaging 입니다. 활성 흔적은 17일차에 유해 자극에 노출되었을 때 개별 세포의 세포내 칼슘 동원이 증가했음을 나타냅니다. 이는 세포가 17일 이내에 기능성 통각수용체 표현형을 갖게 됨을 나타냅니다.

9. Calcium imaging of ioSensory Neurons demonstrates activity following treatment with a TRPM3 agonist
TRPM3 agonist인 CIM0216을 사용한 치료에 반응하여 칼슘 동원을 표시하는 ioSensory Neuron의 영상입니다.
