특징
- Functional human iPSC-derived astrocytes 입니다.
- 주요 성상세포 마커인 SOX9, EAAT1, S100B, Vimentin을 발현합니다.
- 식세포 작용, cytokine 분비, co-culture에서의 신경 활동 조절이 가능합니다.
사양
| Starting
material |
Human iPSC line |
| Donor |
Caucasian adult male (skin
fibroblast) |
| Format |
Cryopreserved cells |
| Differentiation
method |
opti-ox™ cellular reprogramming |
| Karyotype |
Normal (46, XY) |
| Vial size |
Small: 1 x 10^6 viable cells |
| Recommended
seeding density |
30,000 cells/cm2 |
| Seeding
compatibility |
6, 12, 24, 96 and 384 well plates |
| Quality
control |
Sterility, protein expression (ICC),
gene expression (RT-qPCR) |
적용
- Neurodegenerative disease modelling
- Drug screening & development
- Neuropharmacology
- Neuroinflammation research
- Biomarker discovery
프로토콜
ioAstrocytes는 냉동 보존 형식으로 제공되며 권장 배지에서 재생 시 빠르게 성숙되도록 프로그래밍되어 있습니다.
Phase 0 단계의 세포를 수령 후, Phase 1, 2, 3 과정을 거쳐 실험에 바로 이용합니다.
1) Phase 0 : Induction (bit.bio에서 Induction 후 제공)
2) Phase 1 : 고객이 세포를 수령 후 plating 하여 안정화시키는 기간 (2일)
3) Phase 2 : 세포가 성숙하는 기간 (7일)
4) Phase 3 : 세포를 유지하는 기간 (7일)
참고 데이터
1. Easy-to-use co-culture protocol for ioAstrocytes with ioGlutamatergic Neurons
2. Modulation of neuronal activity in co-culture
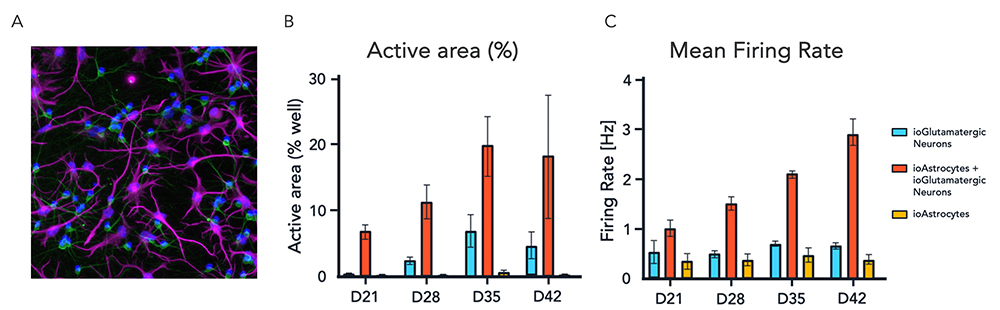
(A) Immunocytochemistry image of ioAstrocytes and ioGlutamatergic Neurons in co-culture; staining shows expression of pan-neuronal marker MAP2 (green), astrocyte marker S100B (purple) and DAPI nuclear staining (blue). (B and C) High-density multi electrode measurements of the neuronal activity of mono- and co-cultures of ioGlutamatergic Neurons and ioAstrocytes, showing the active area (% of well) and mean firing rate (Hz) at different time points. ioAstrocytes were directly derived from iPSC after a 10-day reprogramming protocol (D10), and then harvested to seed with Day 0 (D0) ioGlutamatergic Neurons to establish their co-cultures. D10 ioAstrocytes and D0 ioGlutamatergic Neurons were also used to generate the respective mono-cultures.
3. ioAstrocytes acquire a stellate astrocyte morphology from day 8

ioAstrocytes acquire a stellate shape with branched, elongating processes that continue to intensify. Images captured on an Incucyte at day 0 and days 2, 4, 8, 15 & 22 post-thaw. 10x magnification, 400 µm scale bar.
4. ioAstrocytes secrete cytokines in response to stimulation
MSD multiplex immunoassay measuring whether ioAstrocytes are able to secrete a range of cytokines upon treatment with various proinflammatory stimuli. ioAstrocytes were treated with 3 different proinflammatory cocktails (T) or vehicle (V) at day 15 post-thaw and after 24 hours media samples were collected to measure the concentration of cytokines in the media.
The proinflammatory cocktails induce the secretion of most of the cytokines relative to the vehicle treated cells. Overall, the ioAstrocytes display the expected responses to the three distinct cocktails, including a strong response of Interleukin 6 (IL-6), known to be involved in neuroinflammation. Note that IFNg or IL-1b are present in two of the inflammatory cocktails and, therefore, the presence of these cytokines in the media will lead to high signals upon their detection and interfere with the measurements of their secreted forms.
5. RT-qPCR shows gene expression of key astrocyte markers
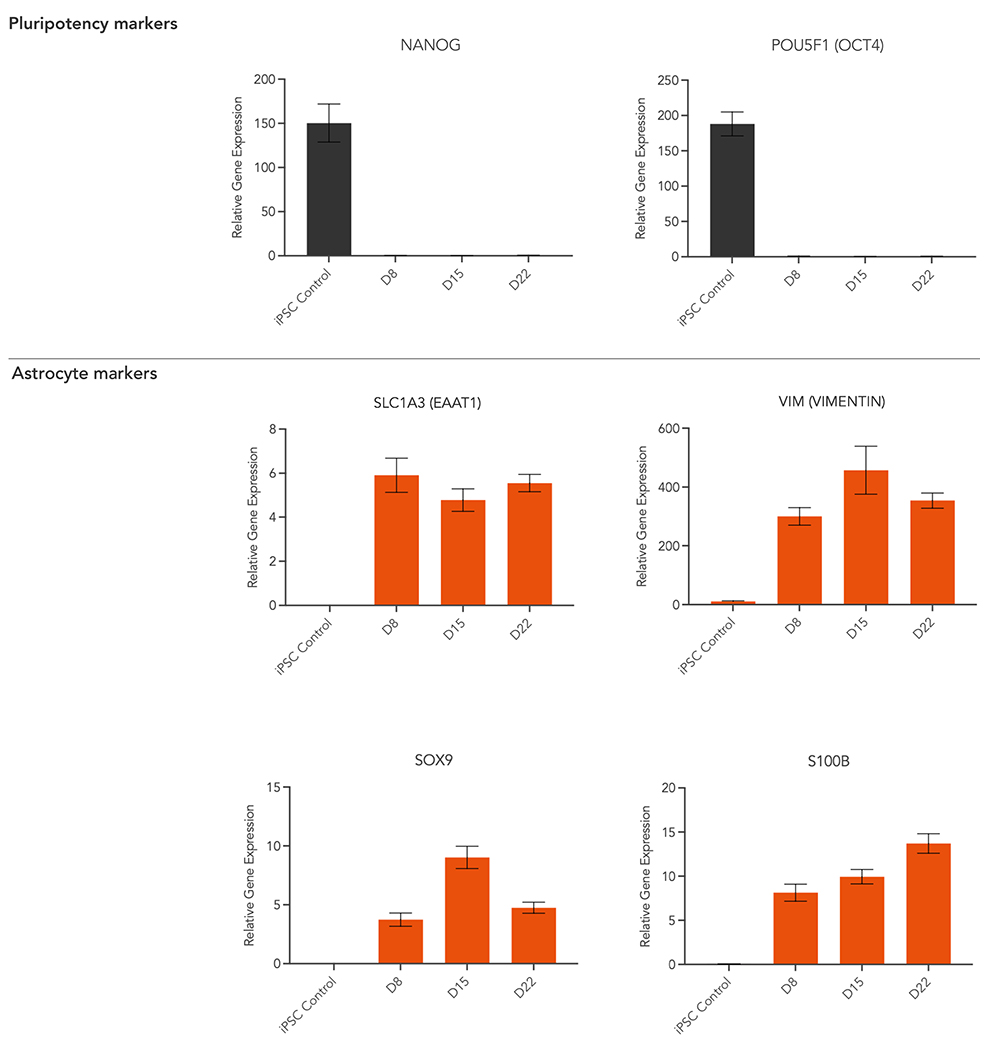
RT-qPCR data showing expression of key astrocyte markers EAAT1, SOX9, S100B and Vimentin (VIM) at four different timepoints (iPSC & D8, D15, D22). ioAstrocytes show expression of key markers from as early as day 8. Pluripotency markers POU5F1 (OCT4) & NANOG) are downregulated.
The SLC1A3 gene codes for the EAAT1 protein (Excitatory Amino Acid Transporter 1). Playing crucial roles in the regulation of glutamate neurotransmission, maintaining neuronal health and protecting against excitotoxicity.